Recommendation Tips About How To Find Out Moles

So a mole of water (h2o) has a mass of 18 g.
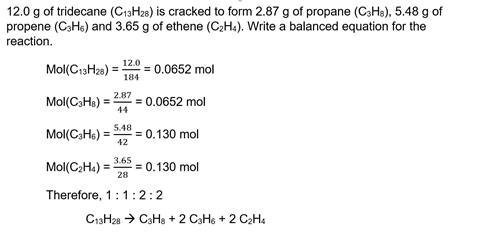
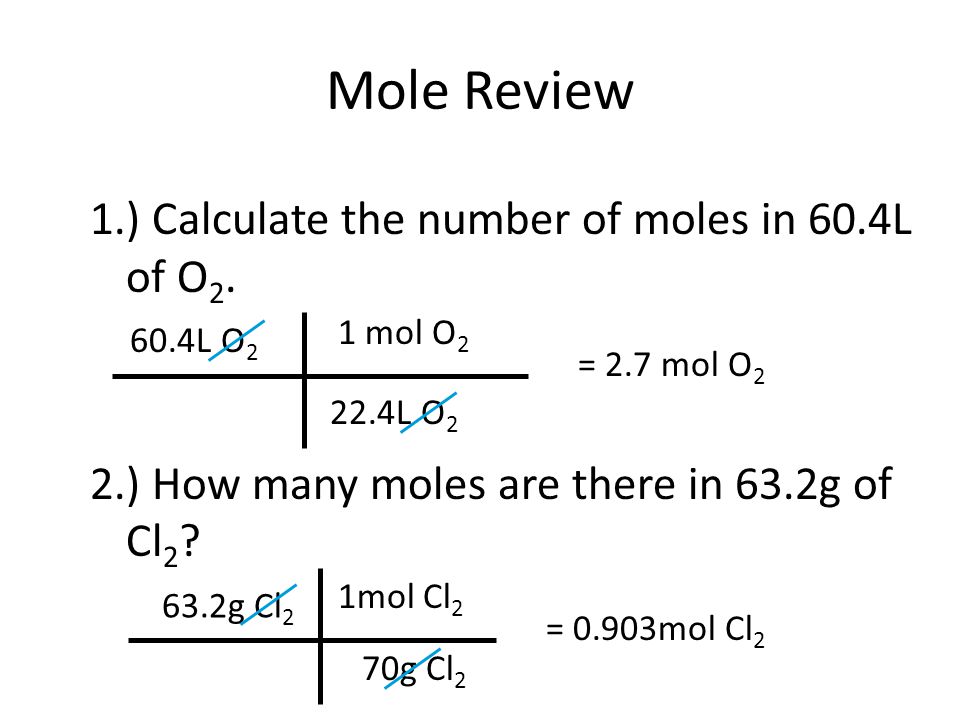
How to find out moles. A mole of carbon dioxide. Number of moles = mass ÷ relative formula mass this can be rearranged to find the mass if the number of moles and molar mass (its relative formula mass in grams) are known. Number of moles formula is.
Finding the moles of product from moles of reactant using a chemical equation example: Mass of one mole mno2 = 86.94g. The number of moles formula is given as follows:
The formula for number of moles. N = m / m where n = number of moles m =. Mole}\) uses of mole concept.
The given chemical reaction is not balanced since the. Avogadro’s number represents a mole ( often abbreviated mol ), and it states that a mole of a substance has (6.022×1023) or 6.022×10^23 molecules per mole or atoms. Unbalanced chemical reaction step 1:.
Number of moles = 95. A stands for asymmetrical shape, which is generally the shape of. Mass of mno2 = 95g.
Ask me questions on facebook: Number of moles = \(\frac {mass\; It has a mass that is equal to its relative formula mass.








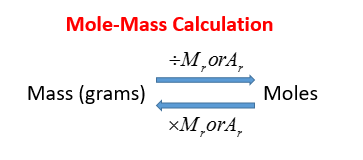

/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)